Pfizer Seeks FDA Approval; Russia Warns Drinking Alcohol May Affect Vaccine Takers
The race for the COVID-19 vaccine does not end in the formulation and clinical trials of the drug.
Before the drug from a pharmaceutical company becomes readily available for the public, the United States Food and Drug Administration (FDA) needs to review and the clinical trials result of the drug such as side effects and the efficacy.
The Emergency Use Authorization (EUA) is the main goal of pharmaceutical companies. According to FDA, EUA helps in strengthening the nation's public health protection against Chemical, Biological, Radiological, and Nuclear Threats (CBRN) through enabling the availability and usage of medical countermeasures (MCMs) needed during public health emergencies.
Now that Pfizer and BioNTech's mRNA vaccine demonstrates 95% efficacy, an advisory panel to the US FDA recommends the EUA of the mRNA vaccine from the said companies.
ALSO READ: Says Success of its COVID-19 Vaccine Trial Signals Breakthrough in Battle Against the Virus
Panel Recommendation
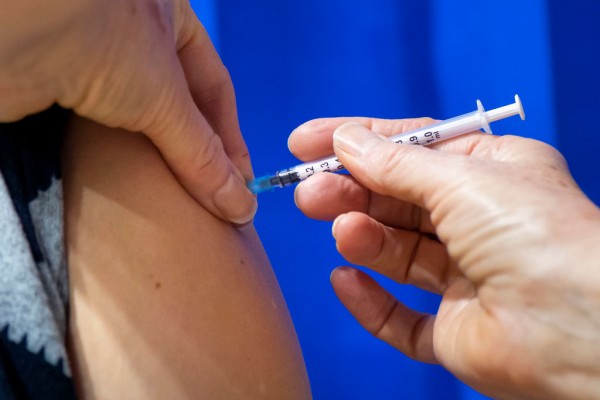
A close-up of a syringe containing a Pfizer-BioNTech Covid-19 vaccine as it is given to a patient at Cardiff and Vale Therapy Centre on December 8, 2020 in Cardiff, Wales. Wales joined the other UK nations in rolling out the covid-19 vaccine on Tuesday, a rare moment of coordination after months of disjointedness in the four nations' pandemic response. Wales introduced a 17-day "firebreak" lockdown in October and November to suppress the surge in covid-19 cases, but infections have continued to rise.
The Guardian reports that the recommendation is anticipated to trigger the first approval of a COVID-19 vaccine to be used imminently in the United States.
The analysis of the FDA also adds that the vaccine has been shown to cause reactions usually lasting a few days. However, according to NPR, the federal agency's analysis also finds no specific safety concerns identified that would preclude the issuance of an EUA.
It can be remembered that Pfizer earlier announced that the pharmaceutical company submitted their vaccine for FDA approval on November 20, and has initiated their submission in neighboring countries such as Canada, Australia, Europe, Japan, United Kingdom. The company adds in their release that they are planning to immediately submit their applications to other regulatory agencies around the globe.
The Guardian mentions an editorial from New England Journal of Medicine, where Dr. Eric Rubin a co-author of the said material notes that most vaccines have taken decades to develop, but this one [mRNA vaccine] is likely to move from outset to large scale implementation within a year.
READ ALSO: Pfizer's COVID-19 Vaccine: Why It Needs To Be Kept Beyond Freezing Point?
Sputnik Vaccine and alcohol

A man finishes a pint of beer outside the City Arms on December 4, 2020 in Cardiff, Wales. Following a firebreak period that ran from October 23 to November 9 the Welsh Government have introduced new rules which will prevent pubs, restaurants and cafes from selling alcohol at any time from 6pm on Friday. The rules will be reviewed on December 17.
Alongside Moderna and Pfizer, another vaccine from Russia runs at the same pace. According to Science Mag, the Gamelaya National Center of Epidemiology and Microbiology reports that interim analysis of a large-scale trial reveals 92% efficacy for the Sputnik V vaccine.
However, a health official warns people that who will get vaccinated by Sputnik V vaccine to give up alcohol for almost two months, according to Reuters.
The Jerusalem Post reports that the warning garnered backlash from the Russian community. In a detailed number of days, the head of the consumer health watchdog Anna Popova in a radio station interview notes that people should stop drinking alcohol at least two weeks before getting the first two injections. She furthers in The Jerusalem Post that the abstain shall continue for another 42 days.
The reason
Popova adds in the Express that if alcohol is a strain in the body and may affect the health and the immune response of the body. She adds that alcohol abstinence would allow the vaccine to work at its best.
With the long-awaited EUA approval of Pfizer's COVID-19 vaccine and the claims of Ms. Popova regarding the alcohol drinking, it is better to take everything in moderation and submit to healthier ways the body will be needed in working with the mRNA vaccine.
READ NEXT: Sputnik V COVID-19 Vaccine's First Batch, Ready for Phase 2, 3 Clinical Trials
Check out more news and information on Pharmaceutical Companies and COVID-19 on MD News Daily.
Dec 11, 2020 06:44 AM EST





